Abstract
Introduction: Post-remission therapy (PRT) for acute myeloid leukemia (AML) remains an area of debate. Autologous stem cell transplantation (AutoSCT) is listed as a possible PRT in patients with intermediate risk AML in the 2017 European Leukemia Network Recommendations. Our center has frequently performed AutoSCT for patients with AML and we aimed to retrospectively compare this strategy with allogeneic SCT (AlloSCT) and consolidation chemotherapy (CMT).
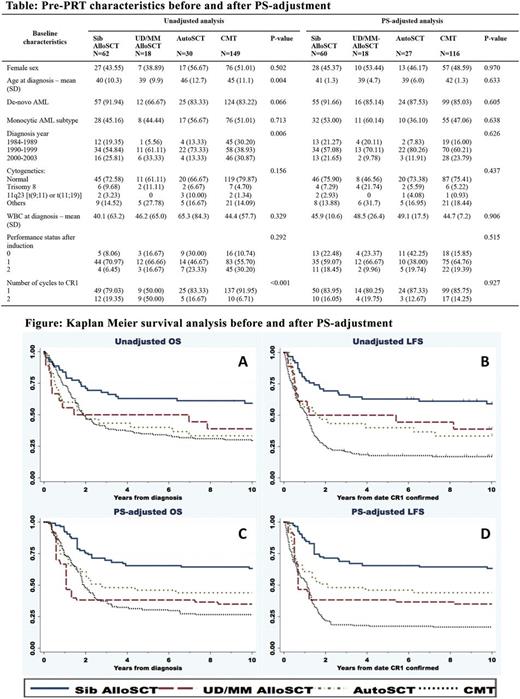
Methods: We compared overall survival (OS) and leukemia free survival (LFS) using propensity score (PS) adjusted analysis of patients receiving PRT with: AutoSCT, matched sibling (Sib) AlloSCT, unrelated/mismatch (UD/MM) AlloSCT and CMT. We included patients diagnosed between 1984-2003 (period of AutoSCT at our centre) in first complete remission (CR1) following induction CMT. The 2010 Medical Research Council cytogenetic classification [Grimwade D. Blood 2010;116(3):354-65] was used to define intermediate risk patients. PS-adjusted analysis was performed with marginal mean weighting through stratification (MMWS) method [Hong G. Psych Methods 2012;17(1):44-60.], accounting for sex, de-novo vs. secondary, year of diagnosis, age, chromosome, performance status and high risk features based on prior institutional guidelines (monocytic subtypes, white blood cell > 50x109/L and > 1 cycle to CR1). In brief, log(PS) were calculated from multinomial regression. We excluded cases with log(PS) outside common support area (20% standard deviation from minimum and maximum of each group). Log(PS) were then stratified into 6 strata. MMWS weight was the product of inverse proportion of treatment in each stratum multiplied by proportion of treatment in the whole population. Data was censored at failure or at 10 years. Unadjusted survival analysis was performed using Kaplan Meier method and multivariate logistic regression, and adjustment was also performed by MMWS weight.
Results: We identified 259 patients (62 Sib-AlloSCT, 18 UD/MM-AlloSCT, 30 AutoSCT and 149 CMT). Baseline pre-PRT characteristics were used for PS calculation before and after weight adjustment (Table). All SCTs were performed with myeloablative conditioning regimens, most being busulfan-cyclophosphamide based. Median follow-up time for patients who survived beyond 1 year was 6.5 years. Unadjusted OS and LFS were as shown in Figure panel A and B. We excluded 38 patients (2 Sib-AlloSCT, 3 AutoSCT and 33 CMT) based on the PS calculation. The pre-PRT characteristics were well balanced after PS adjustment. Adjusted OS at 2 and 5 years were: Sib-AlloSCT 75.3% and 65.5%; UD/MM-AlloSCT 38.2% and 38.2%; AutoSCT 60.7% and 46.0%; CMT 48.0% and 30.4% (Figure panel C). Adjusted LFS were: Sib-AlloSCT 71.4% and 65.5%; UD/MM-AlloSCT 38.2% and 38.2%; AutoSCT 50.8% and 46.0%; CMT 21.9% and 17.4%, respectively (Figure panel D). Adjusted cumulative incidence of relapse and non-relapse mortality (considered as competing risks) at 5 years were: Sib-AlloSCT 17.2% and 20.9%; UD/MM-AlloSCT 32.6% and 33.3%; AutoSCT 48.4% and 10.8%; CMT 80.5% and 10.9%. Adjusted multivariate HR, 95% confidence interval (CI) and p-value for OS, considering CMT as reference, were: Sib-AlloSCT [0.34, 0.19-0.61, <0.001], UD/MM-AlloSCT [1.03, 0.40-2.66, 0.953] and AutoSCT [0.71, 0.33-1.51, 0.374], respectively. Adjusted multivariate HR, 95%CI and p-value for LFS were Sib-AlloSCT [0.22, 0.13-0.37, <0.001], UD/MM-AlloSCT [0.62, 0.27-1.43, 0.262] and AutoSCT [0.45, 0.21-0.96, 0.038], respectively.
Conclusion: Our results show that intermediate risk AML patients who underwent Sib-AlloSCT in CR1 had the best outcomes. AutoSCT significantly improved LFS, in comparison to CMT. However, this benefit did not result in a significant improvement in OS, possibly due to subsequent SCT in the CMT group after relapse. Limitations of our findings include a lack of molecular data and the older time period of our cohort. In a more contemporary cohort, we anticipate that advancements in donor selection and supportive care might improve outcomes with AlloSCT, particularly for the unrelated donor group. Despite this, our results support AutoSCT as a valid option for intermediate risk AML patients who lack an HLA-matched sibling. Further studies integrating molecular genetics and minimal residual disease are needed to guide the selection of patients for AutoSCT who are likely to gain the greatest benefit.
Hogge: Novartis, Roche, and Sanofi: Consultancy. Sutherland: Janssen: Honoraria. Gerrie: Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Lundbeck: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.